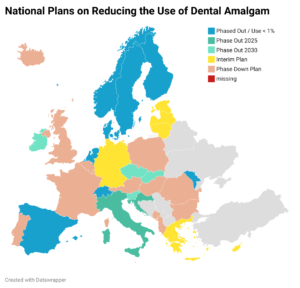
Updated: Manufacturers leaving the business of Dental Amalgam in Europe
Since the new Medical Devices Regulation (MDR 2017/745) entered into force on May 26, 2021, the legal safety requirements for dental amalgam capsules have increased significantly, why manufacturers start leaving the European business…