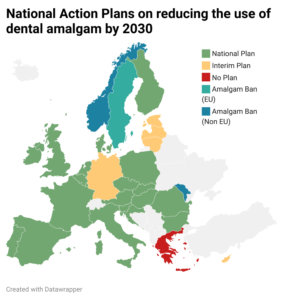
Adoption by the Council: Dental Amalgam will be banned by January 2025
Brussels, 30 May 2024: With the adoption by the Council, the phase-out of dental amalgam by January 2025 has been finalized. The European Parliament had already given its approval on April 10 with a majority of 98%. After being signed by the Presidents of the European Parliament and the Council, the legal act will soon be published in the Official Journal of the European Union and enter into force…